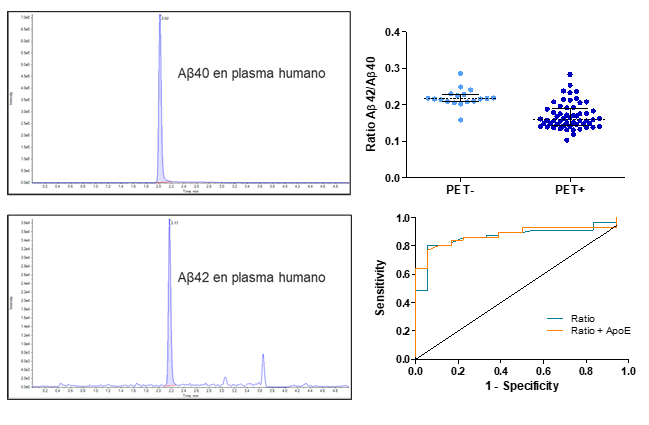
Determinación de la ratio Aβ42/Aβ40 mediante ABtest-MS en un subgrupo de 74 muestras de pacientes con MCI del ensayo de fase II de ABvac40 (vacuna activa anti-Aβ40).
La ratio Aβ42/Aβ40 en plasma difiere significativamente entre los individuos PET-amiloide positivos y negativos (P<0,0001).
La ratio Aβ42/Aβ40 en plasma discrimina PET-amiloide positivos y negativos con alta exactitud (AUC = 0,872; 0,881 para el modelo ajustado para el genotipo ApoE) y valores equilibrados de sensibilidad (80.4%) y especificidad (94,4%)