ABtest-MS is a new option within ABtest-Service based on mass spectrometry that allows the simultaneous determination of Aβ1-40 and Aβ1-42 from just 250 microliters of plasma.
ABtest-MS does not require enzymatic digestion or prior immunoprecipitation of the samples, substantially shortening response time and reducing costs compared to other similar assays.
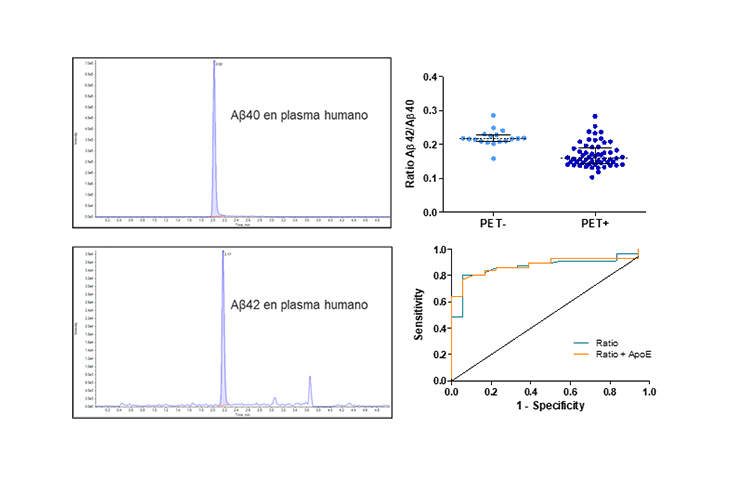
Aβ42/Aβ40 Ratio
Determination of the Aβ42/Aβ40 ratio using ABtest-MS in a subgroup of 74 samples from patients with MCI from the phase II trial of ABvac40 (active anti-Aβ40 vaccine).
The Aβ42/Aβ40 ratio in plasma differs significantly between PET-amyloid positive and negative individuals (P<0.0001).
The Aβ42/Aβ40 ratio in plasma discriminates PET-amyloid positive and negative individuals with high accuracy (AUCROC=0.872; 0.881 for the model adjusted for ApoE genotype) and balanced sensitivity (80.4%) and specificity (94.4%).
Our services
How can we help you?
Contact the Araclon Biotech team.