Numerous results from different laboratories and various assays now show that plasma levels of Aβ40/Aβ42 reflect the brain amyloid burden, considered to be the first pathological change associated with Alzheimer’s Disease (AD)1.
Determining the plasma levels of these peptides could help in the diagnosis and prognosis of AD, especially in people who are still asymptomatic2.
In addition, the use of these plasma tests as pre-screening (triage) tools could make it much easier and cheaper to recruit these individuals into clinical trials of new treatments that seek to modify the course of the disease by halting or delaying the onset of symptoms.

Araclon Biotech has been committed since its foundation to the development of blood biomarkers in order to help in the diagnosis of AD. Along these lines we have developed analysis procedures for the quantification in plasma* (of Aβ40 and Aβ42; peptides (ABtest-Service) whose accumulation in the brain is considered to be the first pathological change related to AD1.

Automated service
ABtest-Service guarantees reliable results from automated analysis by immunoassay (ABtest-IA) or by mass spectrometry (ABtest-MS) under strictly standardized conditions and performed by highly qualified personnel.
ABtest-Service has already been tested in different cohorts globally, with satisfactory results.
We do not sell kits, rather we offer a testing service (ABtest-Service). You only have to send us your plasma (or cerebrospinal fluid) samples*.

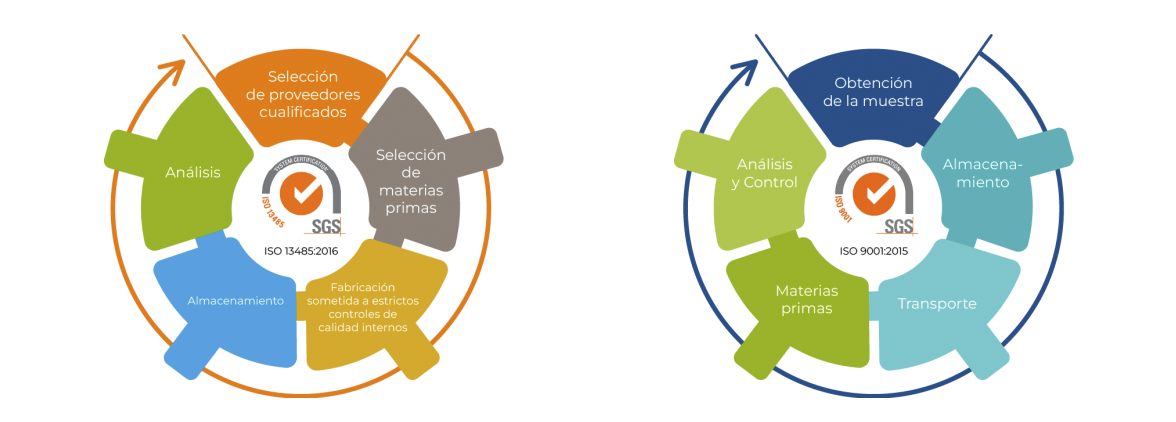
Araclon Biotech is deeply committed to quality, the company has a strict quality control system certified ISO 9001 (2015) and ISO 13485 (2016) and SOPs established for all processes, including supplier auditing.
Reliable and high-quality results
- ABtest-Service guarantees reliable results from automated analysis by immunoassay (ABtest-IA) or mass spectrometry (ABtest-MS).
- It is offered in the USA under the USABtest® registered trademark.
- It can be arranged in two different modalities, ABtest-IA and ABtest-MS.
- Analytically speaking, both procedures, ABtest-IA and ABtest-MS, are fully validated.
- ABtest-IA is currently CE marked in the EU and Investigational Use Only (IUO) labelled in the rest of the world.
References
- Gouras GK, Olsson TT, and Hansson O. β-amyloid Peptides and Amyloid Plaques in Alzheimer’s Disease. Neurotherapeutics. 2015; 12 (1):3-11. https://pubmed.ncbi.nlm.nih.gov/25371168
- Bateman RJ et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s Disease. N Engl J Med. 2012; 367:795-804. https://pubmed.ncbi.nlm.nih.gov/22784036
*Frozen plasma samples, ideally at -80°C, should be submitted to our laboratory. ABtest-Service only needs 300 microlitres of plasma for the determination of the Aβ42/Aβ40 ratio. Whole blood (minimum sample volume 500 microlitres) collected in EDTA K2 tubes is also acceptable if refrigerated immediately after collection and delivered to our laboratory within 30 hours. Our analyses are validated for plasma and cerebrospinal fluid, and for work with blood serum, other biological fluids and cell culture media.
Our services
How can we help you?
Contact the Araclon Biotech team.